A candidate glycoconjugate vaccine containing Vi-polysaccharide of Salmonella Typhi, chemically conjugated to a conserved outer membrane protein T2544 expressed by multiple Salmonella species. The solvent used for the vaccine preparation was PBS 1X .
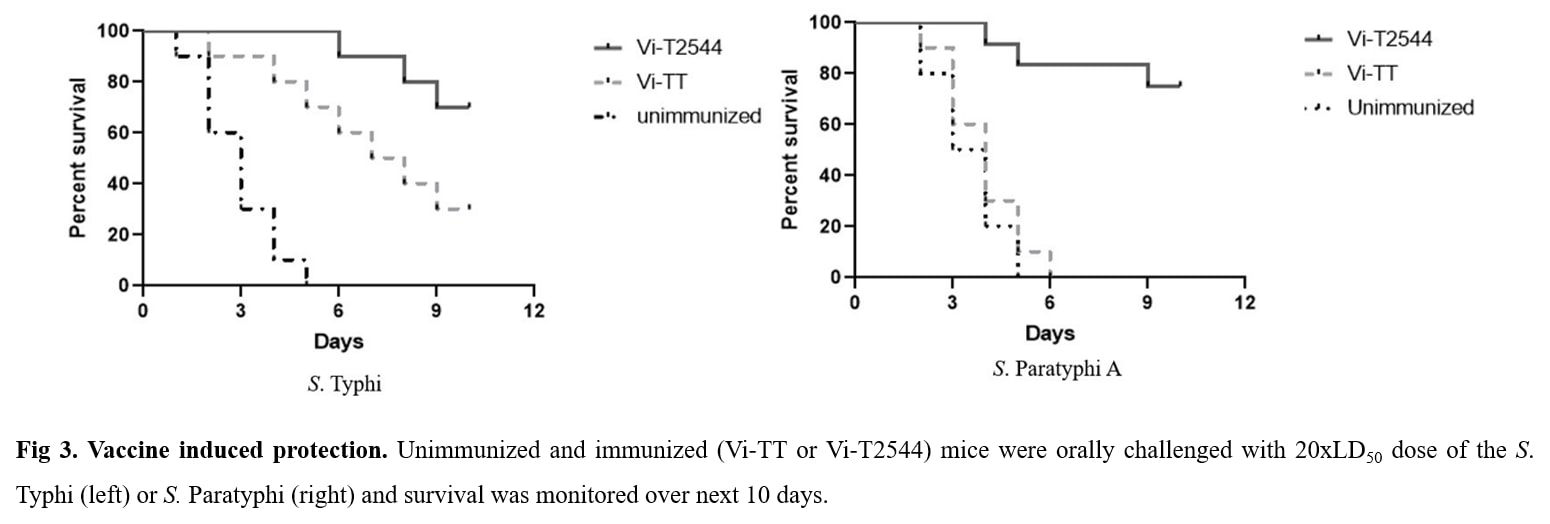
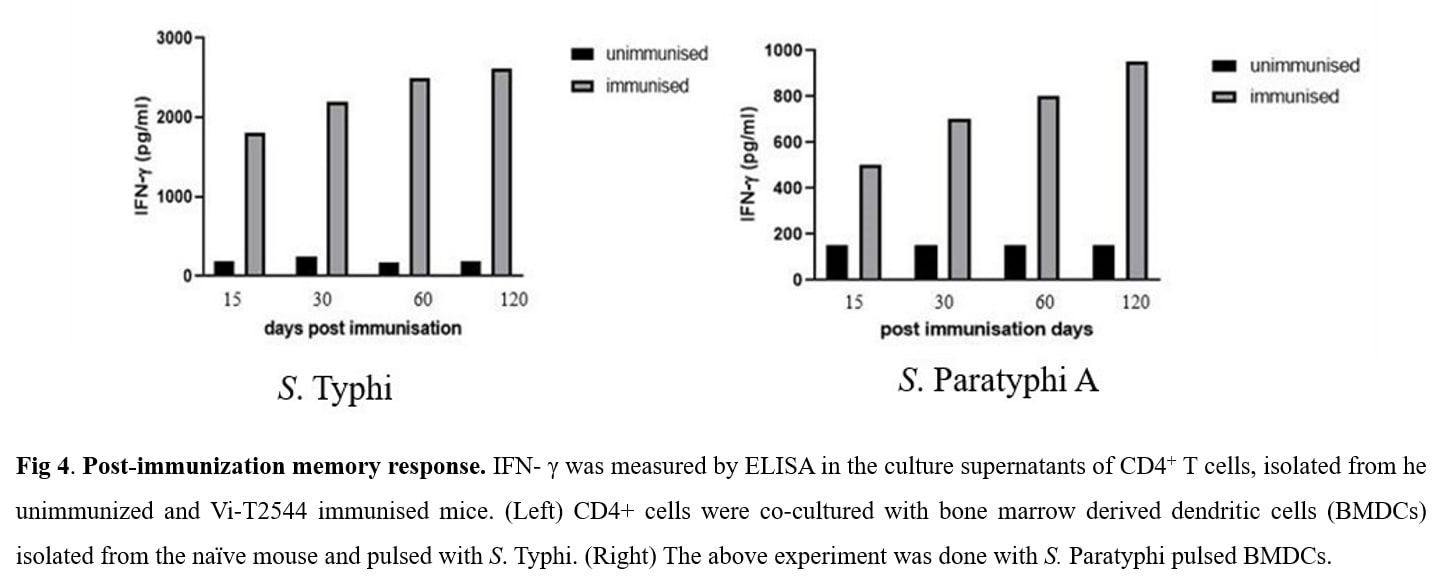
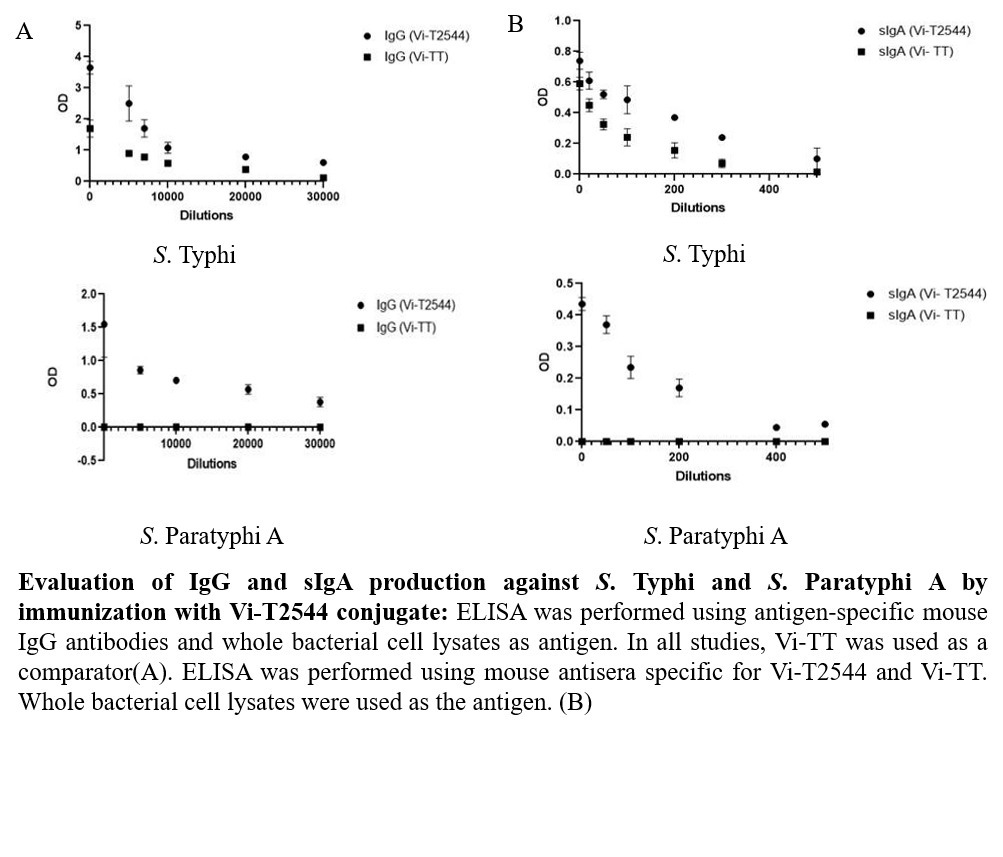
USP: The candidate glycoconjugate vaccine Vi-T2544 is safe, easy to prepare and strongly immunogenic in mice with induction of serum and mucosal intestinal antibodies and cell-mediated immune response. In addition, Vi-T2544 induced significant number of memory CD4+ T cells that secrete IFN-?. This vaccine induced higher antibody titers compared with Typbar TCV vaccine. Unlike the existing typhoid vaccines, it showed dual specificity with protection against both S. Typhi and S. Paratyphi A infections in mice.
- Innovator's Name
- Product/Technology Description
National Institute of Cholera and Enteric Diseases
- Product Name: A candidate glycoconjugate vaccine containing Vi-polysaccharide of Salmonella Typhi, chemically conjugated to a conserved outer membrane protein T2544 expressed by multiple Salmonella species. The solvent used for the vaccine preparation was PBS 1X .
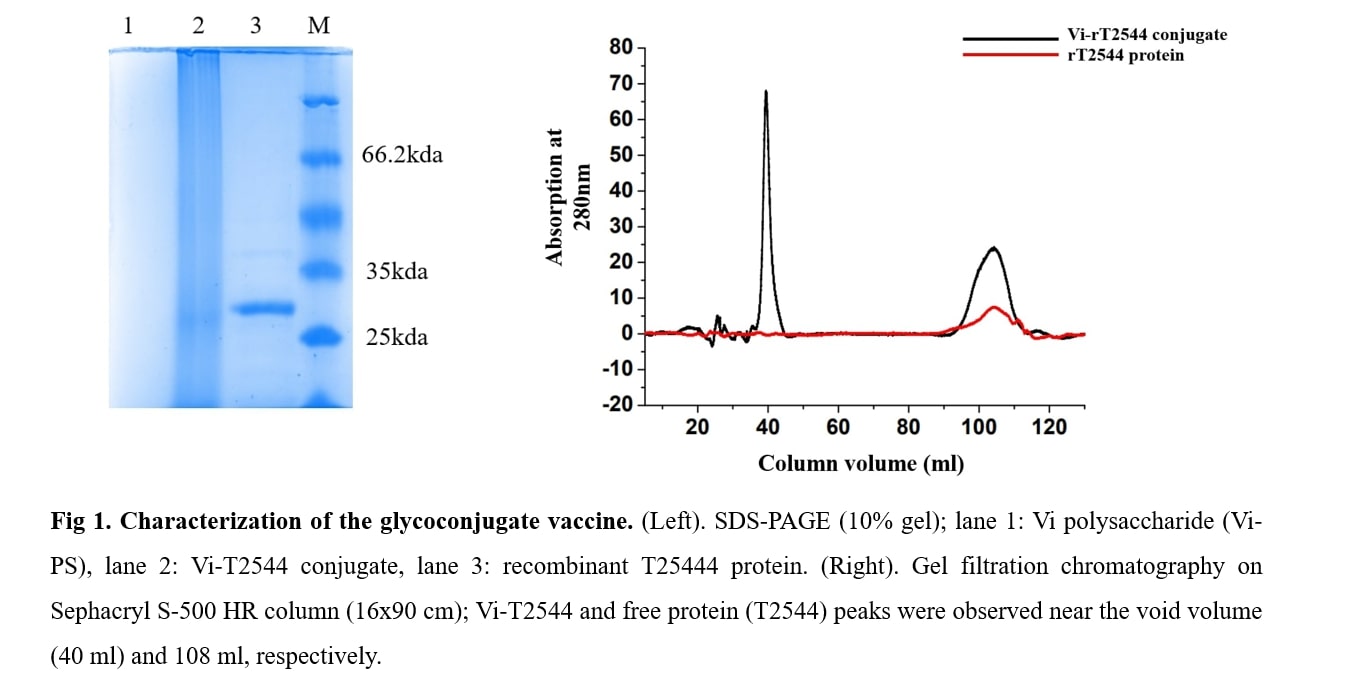
- Product Title: Recombinant T2544 expressed in E. coli was purified by ion exchange chromatography and conjugated to purified Vi-PS by ADH linker using EDAC chemistry. The glycoconjugate was purified by gel filtration chromatography and used for immunization of BALB/c mice 25?g/dose x 3 subcutaneous doses,12 days apart . Vi-tetanus toxoid conjugate vaccine Typbar TCV, Bharat biotech, India was used in the same dosing schedule as a comparator for the study. Immunogenicity was measured by serum IgG and intestinal secretory IgA titers and the induction of CD4+ T-cell memory. Vaccine protective efficacy was evaluated by challenges with 20xLD50 dose of S. Typhi or S. Paratyphi A of the immunized mice in an iron overload model of oral infection. Toxicity of the vaccine formulation was evaluated in our laboratory by a WHO-recommended battery used for new vaccines and also from an external accredited laboratory Shriram Institute for Industrial Research, New Delhi .
- Description:
- Unique Selling Point: The candidate glycoconjugate vaccine Vi-T2544 is safe, easy to prepare and strongly immunogenic in mice with induction of serum and mucosal intestinal antibodies and cell-mediated immune response. In addition, Vi-T2544 induced significant number of memory CD4+ T cells that secrete IFN-?. This vaccine induced higher antibody titers compared with Typbar TCV vaccine. Unlike the existing typhoid vaccines, it showed dual specificity with protection against both S. Typhi and S. Paratyphi A infections in mice.