Development of novel therapeutic regimen for treating drug sensitive and resistant strains including MDR and XDR strains of tuberculosis involving Pranlukast PRK , a novel anti-tubercular agent in combination with rifampicin RIF and isoniazid INH
USP: PRK clears Mtb in the lungs of guinea pig and mice at a clinically approved dosage and without any harmful side effects known.
- Innovator's Name
- Product/Technology Description
Indian Institute of Science Bangalore
- Product Name: Development of novel therapeutic regimen for treating drug sensitive and resistant strains including MDR and XDR strains of tuberculosis involving Pranlukast PRK , a novel anti-tubercular agent in combination with rifampicin RIF and isoniazid INH
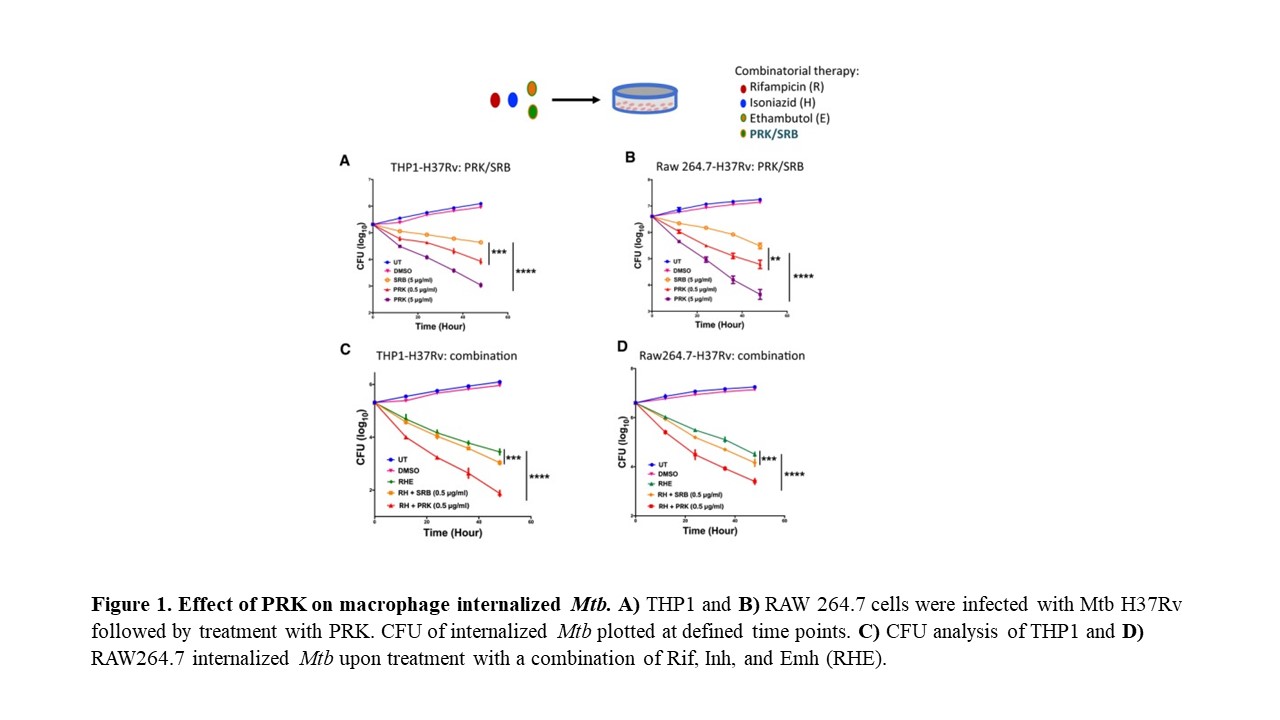
- Product Title: We discovered a novel allosteric site at the surface of ArgJ tetramer of Mtb. Identified in silico potential inhibitors for it from FDA approved drugs Inhibitors were validated by inhibition of the purified enzyme Pranlukast inhibited Mtb growth in cultures, in macrophage infection model and in mice in vivo. PRK inhibits growth of the MDR snd XDR strains of Mtb Metabolomics efficacy of PRK is related to its ability to dysregulate arginine and proline metabolism, aromatic amino acid metabolism, purine metabolism and oxidative phosphorylation Proteomic studies of Mtb infected mouse macrophages treated with PRK shows increase in autophagy and decrease in ROS and inflammation in macrophages Thus, PRK compromises the pathogenic mechanism of Mtb by modulating a number of its virulence strategies in its host. PRK is very effective in guinea pig. Orally administered PRK is more potent than even RIF and combination of RIF and PRK and HREZ+PRK are much more effective. PRK has no toxic side effects on the treated animals Overall PRK contributes positively to healing of infected lungs by mobilizing Arginase positive macrophages
- Description:
- Unique Selling Point: PRK clears Mtb in the lungs of guinea pig and mice at a clinically approved dosage and without any harmful side effects known.