MIQNAF - Nafithromycin Tablet
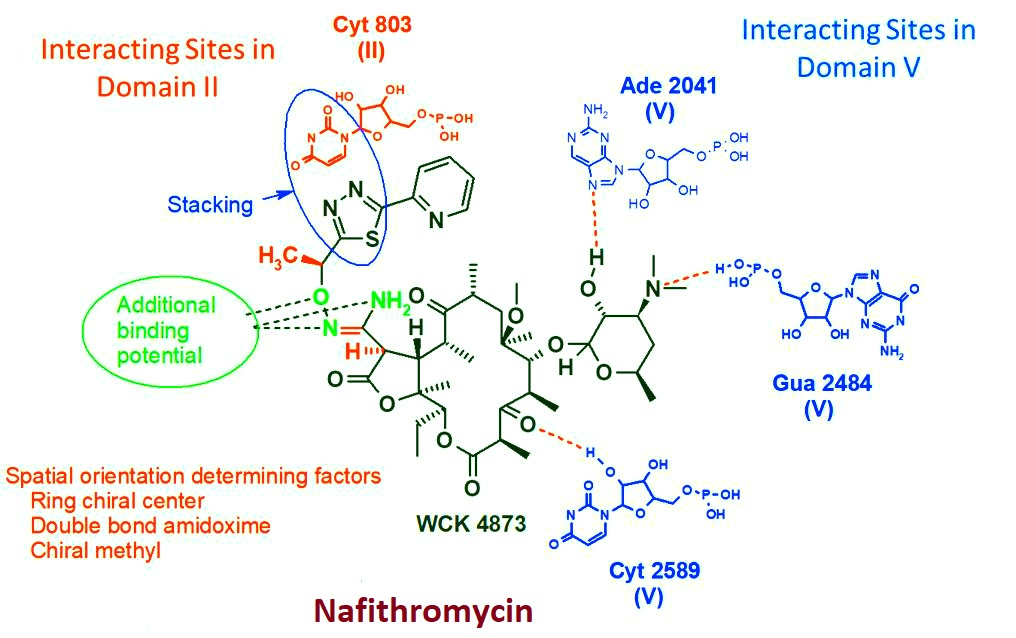
USP: 1. Unique structural features endowed nafithromycin are Binding to two sites on 23S rRNA of bacterial ribosomes that fully overcomes azithromycin and clarithromycin resistance Potent in vitro activity based on dual mechanism of action coupled with bactericidal property and longer post-antibiotic effect Higher oral absorption Best-in-class lung penetration site-of-infection along with high alveolar macrophage & PMN concentrations Sustained, therapeutically relevant levels in the lungs even up to 48 hours after the last dose Favourable anti-inflammatory effects Lack of QT prolongation Hepato-safe Devoid of significant CYP inhibition or induction potential No drug-drug interactions Suitable for all age groups 2.Nafithromycin has undergone a rigorous non-clinical and clinical Phase 1, 2 and 3 development for which studies were conducted in US, EU and India, spanned over 12 years. As India marks the macrolide resistance of 60 among pneumococcal isolates ICMR 2023 data , there is an urge for safer and effective CABP treatment for Indian patients. Nafithromycin, with its superior safety & potent antibacterial activity would successfully address this AMR challenge in India.
- Innovator's Name
- Product/Technology Description
Wockhardt Limited
- Product Name: MIQNAF - Nafithromycin Tablet
- Product Title: MIQNAF
- Description: MIQNAF - Nafithromycin is a novel oral macrolide antibiotic discovered & developed in India after 30 years, offering a first-ever, ultra-short course once-daily, 3-day, safe, monotherapy for the treatment of community-acquired bacterial pneumonia CABP caused by any of the typical and atypical pathogens including those that are multi-drug resistant. India is home to 23 of global burden of CABP. Currently, CABP patients often do not respond well to existing oral antibiotics leading to hospitalization which in turn enhances the risks of hospital-acquired infections and treatment cost and even ICU admission. 20 mortality is reported in CABP patients admitted to ICU. MIQNAF has the potential to prevent such hospitalizations and mortality.
- Unique Selling Point: 1. Unique structural features endowed nafithromycin are Binding to two sites on 23S rRNA of bacterial ribosomes that fully overcomes azithromycin and clarithromycin resistance Potent in vitro activity based on dual mechanism of action coupled with bactericidal property and longer post-antibiotic effect Higher oral absorption Best-in-class lung penetration site-of-infection along with high alveolar macrophage & PMN concentrations Sustained, therapeutically relevant levels in the lungs even up to 48 hours after the last dose Favourable anti-inflammatory effects Lack of QT prolongation Hepato-safe Devoid of significant CYP inhibition or induction potential No drug-drug interactions Suitable for all age groups 2.Nafithromycin has undergone a rigorous non-clinical and clinical Phase 1, 2 and 3 development for which studies were conducted in US, EU and India, spanned over 12 years. As India marks the macrolide resistance of 60 among pneumococcal isolates ICMR 2023 data , there is an urge for safer and effective CABP treatment for Indian patients. Nafithromycin, with its superior safety & potent antibacterial activity would successfully address this AMR challenge in India.