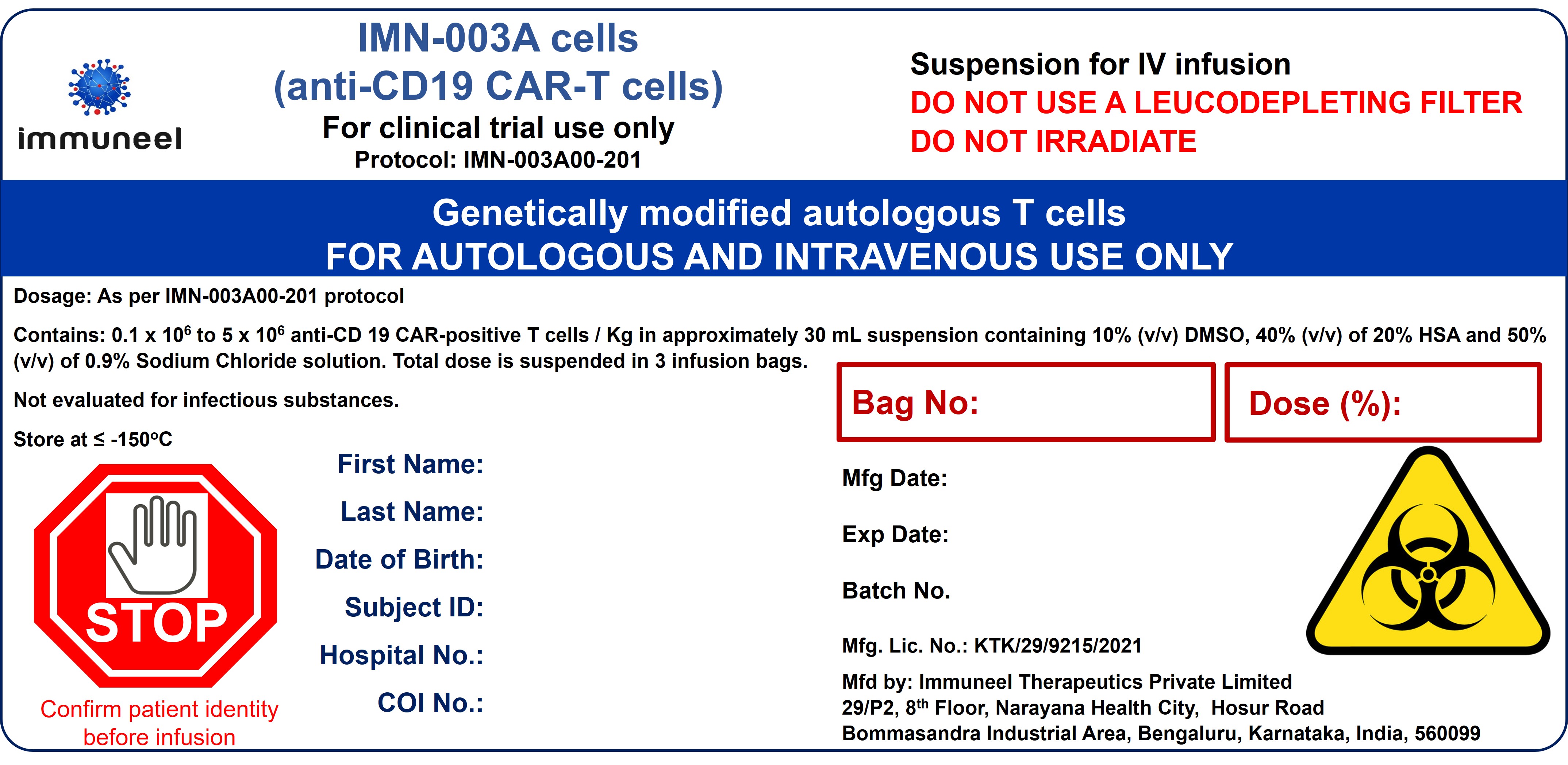
CD19 CAR-T Cell Therapy
Varnimcabtagene autoleucel is an anti-CD19 directed genetically modified autologous Chimeric Antigen Receptor T CAR-T cell therapy for patients with Relapsed / Refractory B Cell Malignancies. It is approved by India regulator for patients with B cell Non-Hodgkins Lymphoma above 18 years. Regulatory approval for B cell Acute Lymphoblastic Leukemia is pending.
USP: Only CD19 CAR-T cell therapy in collaboration with Spain with robust global safety and efficacy data. Approved for hospital use for B cell Acute Lymphoblastic Leukemia in Spain. Unique A3B1 binder with fractionated infusion protocol with proven additional safety.
- Innovator's Name
- Product/Technology Description
Immuneel Therapeutics Pvt. Ltd
Company fully focused on cell and gene therapy solutions. State-of-the-are cGMP manufacturing facility. Highly skilled leadership and human capital.
- Product Name: CD19 CAR-T Cell Therapy
- Product Title: Varnimcabtagene Autoleucel Brand Name: Qartemi
- Description: Varnimcabtagene autoleucel is an anti-CD19 directed genetically modified autologous Chimeric Antigen Receptor T CAR-T cell therapy for patients with Relapsed / Refractory B Cell Malignancies. It is approved by India regulator for patients with B cell Non-Hodgkins Lymphoma above 18 years. Regulatory approval for B cell Acute Lymphoblastic Leukemia is pending.
- Unique Selling Point: Only CD19 CAR-T cell therapy in collaboration with Spain with robust global safety and efficacy data. Approved for hospital use for B cell Acute Lymphoblastic Leukemia in Spain. Unique A3B1 binder with fractionated infusion protocol with proven additional safety.