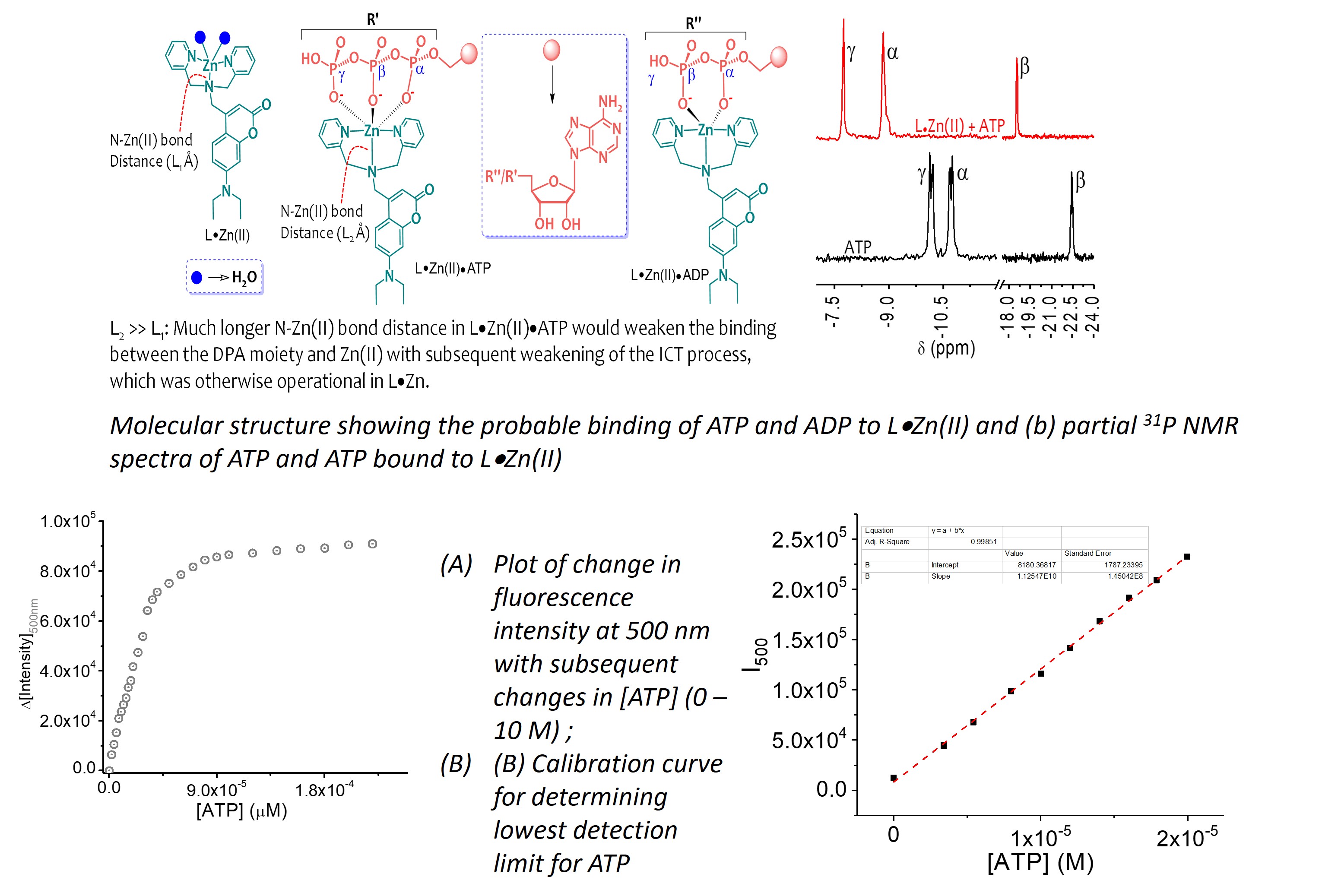
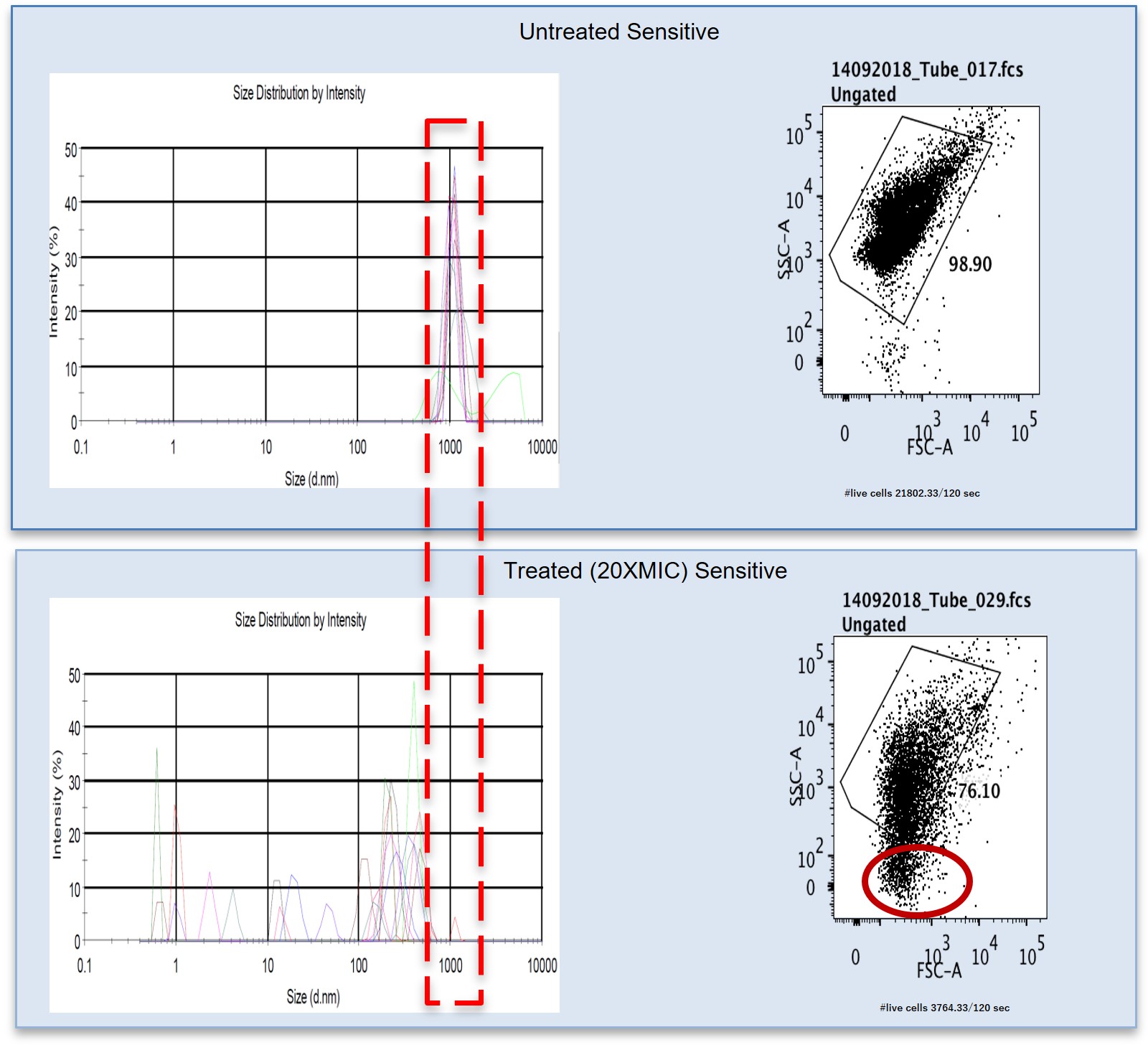
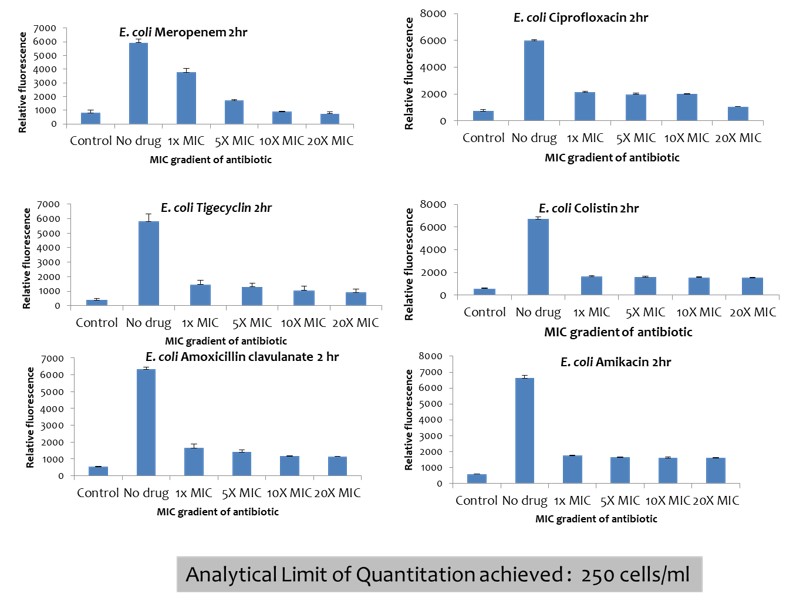
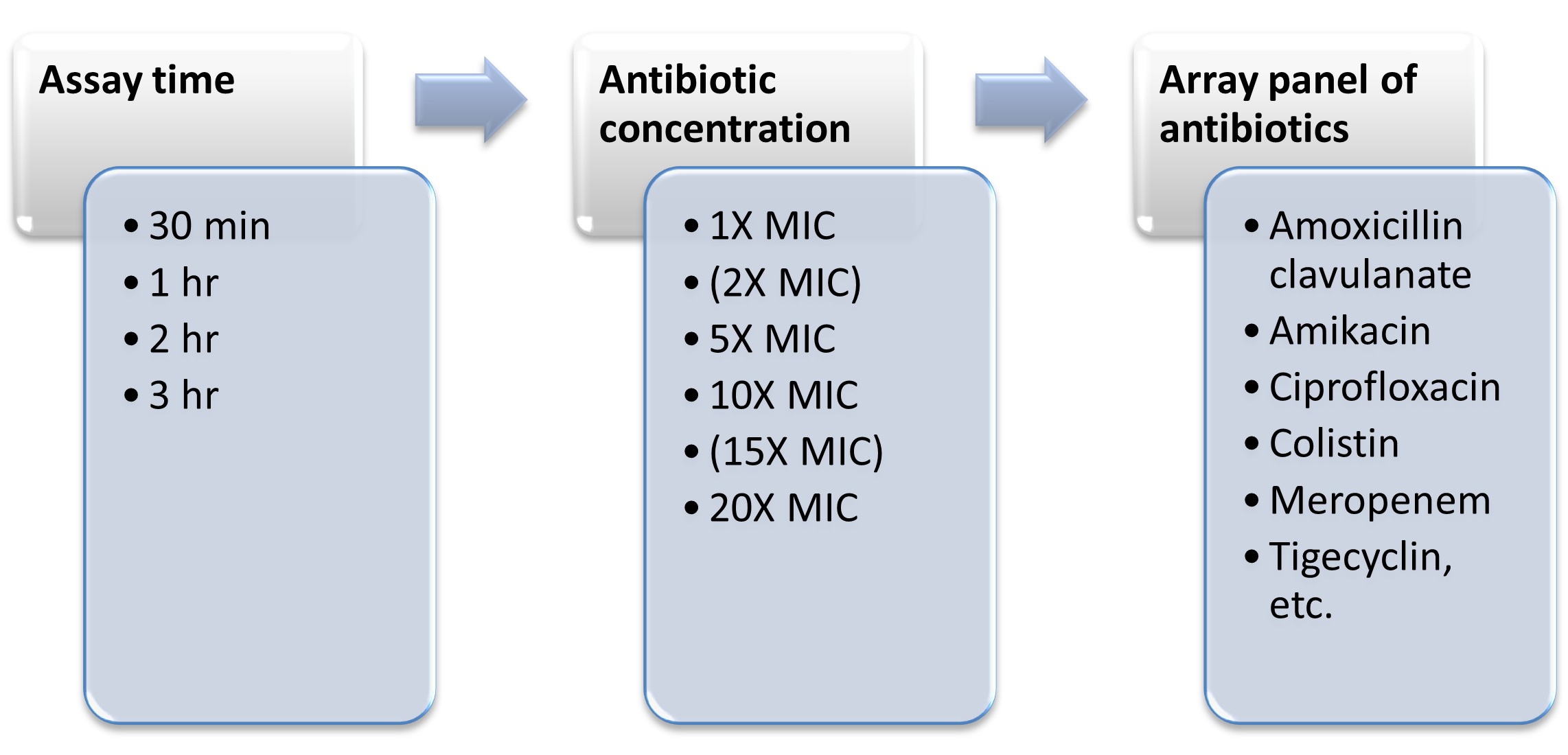
Development and evaluation of a rapid phenotypic antimicrobial susceptibility test for clinical use
USP: This assay can be adapted to any secondary or tertiary level facility for guided clinical decision making for UTI and sepsis management. We hope that this approach will result in a paradigm shift in diagnostic microbiology and will have a significant impact in fighting AMR, as rapid and right diagnosis of resistance/susceptibility is the first major step towards reducing emergence and spread of AMR.
- Innovator's Name
- Product/Technology Description
Translational Health Science and Technology Institute THSTI
- Product Name: Development and evaluation of a rapid phenotypic antimicrobial susceptibility test for clinical use
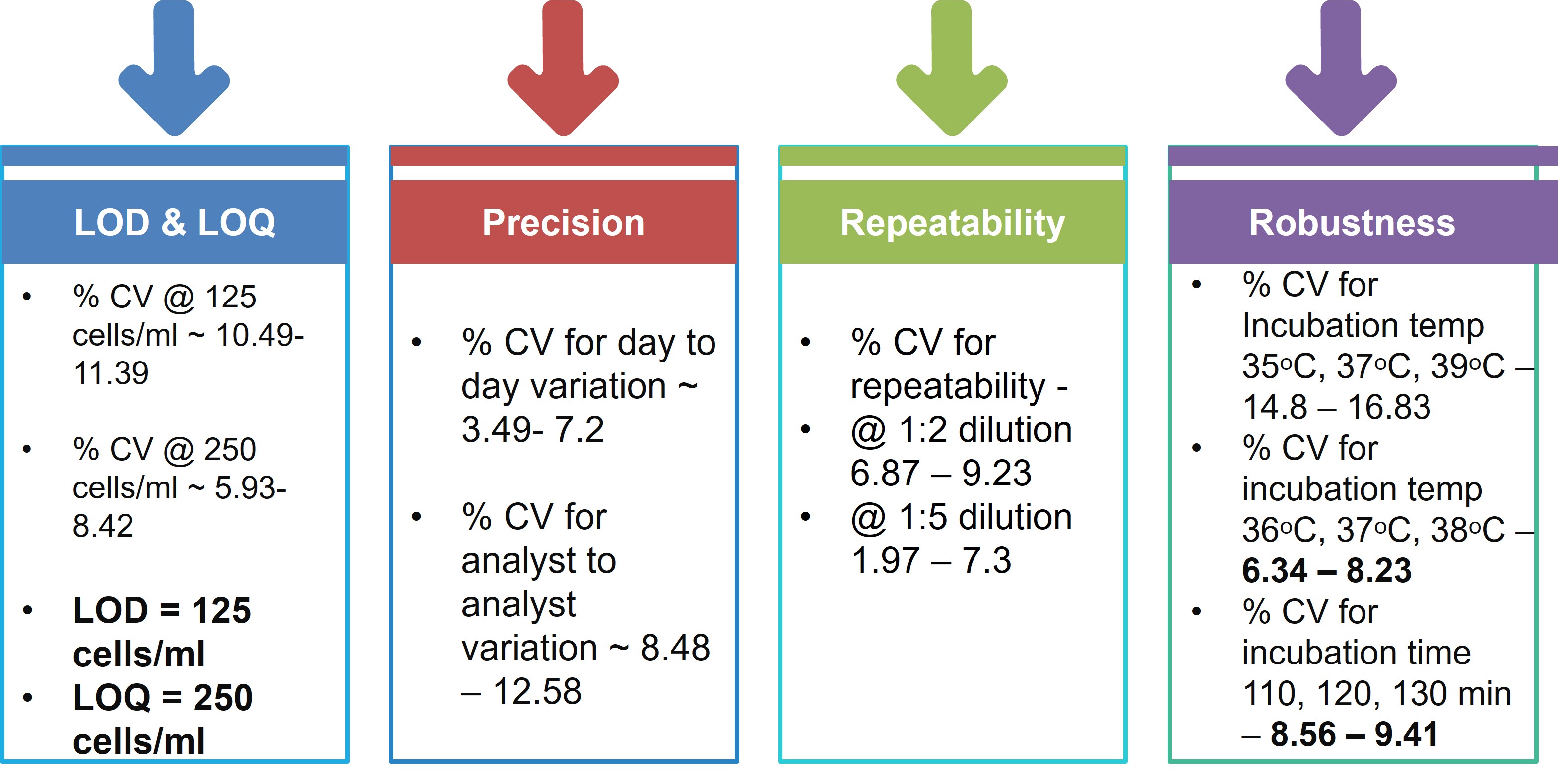
- Product Title: All the required reagents of the diagnostic assay were produced and analytically validated for assay performance. ppGpp sensor was not taken forward since it did not give consistent results. Testing of various micro-well design for assay, select components of assay Quality testing of the assay Developed a self-contained kit Identify isolates from existing clinical isolates in the clinical microbiology lab of partner 100 completed Standard microbiological testing of clinical isolates 100 completed Evaluation and analysis of comparative performance for non-inferiority of the new diagnostic test We aimed to develop the prototype rapid diagnostic kit with all the necessary components and evaluate this kit with clinical isolates of Klebsiella pneumonia and uropathogenic E. coli in this proposal. This assay can be adapted to any secondary or tertiary level facility for guided clinical decision making for UTI and sepsis management. We hope that this approach will result in a paradigm shift in diagnostic microbiology and will have a significant impact in fighting AMR, as rapid and right diagnosis of resistance/susceptibility is the first major step towards reducing emergence and spread of AMR. A real time pilot clinical evaluation is going on in collaboration with AIIMS-Delhi and will lead to further improvement and optimisation of the product in another two years time.
- Description:
- Unique Selling Point: This assay can be adapted to any secondary or tertiary level facility for guided clinical decision making for UTI and sepsis management. We hope that this approach will result in a paradigm shift in diagnostic microbiology and will have a significant impact in fighting AMR, as rapid and right diagnosis of resistance/susceptibility is the first major step towards reducing emergence and spread of AMR.