In reference to the Clinical development of Biologicals (Vaccines and Biosimilars), one of the needs identified by Biotech companies is the challenge in accessing trained and equipped clinical trial sites in the country. Clinical trials are currently an important bottleneck in the product development process as companies and institutions usually have to go through an ad hoc process of finding sites with access to the required population that meet internationally accepted clinical and ethical standards. To solve this bottleneck, National biopharma Mission (NBM) will support the establishment of a clinical trial networks (CTN) and strengthen clinical trial capacity in the country.
To support vaccine development, the mission will support conduct of epidemiology studies of relevant diseases in healthy cohorts in already existing Demographic Surveillance sites (DSS) and further strengthening of these DSS sites for conduct of GCP compliant trials.
CTN Field Sites
|
S. No. |
Name of Sites |
Location |
EHRMP |
|
DHS sites |
|||
|
1 |
Society for Health Allied Research and Education INDIA |
Hyderabad, Andhra Pradesh |
|
|
2 |
Christian Medical College Vellore Association |
Vellore, Tamil Nadu |
|
|
3 |
ICMR- National Institute of Epidemiology |
Tiruvenelveli, Tamil Nadu |
|
|
4 |
The INCLEN Trust International |
Palwal, Haryana |
|
|
5 |
KEM Hospital Research Centre |
Vadu, Maharashtra |
|
|
6 |
Pondicherry Institute of Medical Sciences |
Pondicherry, Tamil Nadu |
|
|
7 |
Maulana Azad Medical College |
New Delhi |
|
|
8 |
Society for Applied Studies |
New Delhi |
|
|
9 |
ICMR-Regional Medical Research Center |
Bhubaneshwar, Odisha |
|
|
10 |
Andhra Medical College |
Vishakhapatnam, Andhra Pradesh |
|
|
11 |
The INCLEN Trust International |
Shillong, Meghalaya |
|
|
Data Management Platform |
|||
|
1 |
The INCLEN Trust International |
New Delhi |
|
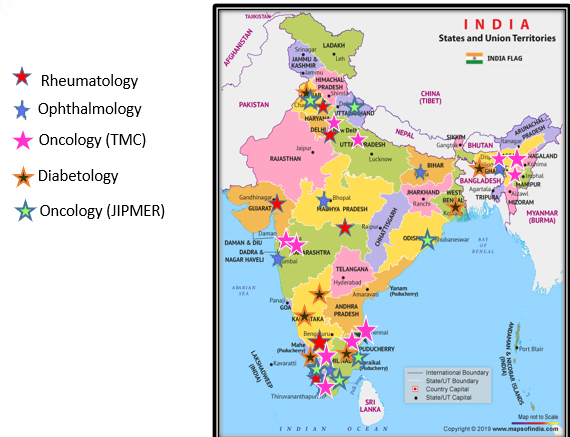
National Biopharma Mission is An Industry-Academia Collaborative Mission of Department of Biotechnology (DBT) for Accelerating Early Development for Biopharmaceuticals; to be implemented by Biotechnology Research Assistance Council (BIRAC). The Mission Programme is a Pan-India Programme with the main aim of making India a hub for design and development of novel, affordable and effective biopharmaceutical products and solutions. This Program would aid in enhancing India’s innovation research and product development capabilities, especially by focusing on development of vaccines, biologics and medical devices for combating public health concerns. Clinical trial is an important step in the product development path requiring multiple trial sites. Indian Biotech companies face numerous challenges in identifying trial sites which have the required infrastructure, capacity, trained manpower, harmonized processes and background data on disease incidence at institutional, local, regional/state, and national levels. The delay in conduct of clinical trials can impact the development timelines of biologics and drugs and thereby delaying the launch of affordable biosimilars for Indian population. To address the above, Department of Biotechnology and National Biopharma Mission’s initiative, Clinical Trial Networks, with Consortia of Hospitals in the areas of Oncology, Ophthalmology, Rheumatology and Diabetology, CHOORD aims to strengthen the capacity to conduct clinical trials in India for products developed in these areas. It comprises of 5 networks of 36 organizations, public and private hospitals, clinics, reputed academic institutions, spread across 18 states of India. Each specialty network comprises of at least 6 hospital-based investigational sites (public and private hospital sites) across different geographical regions of the country. This e-book captures the information about these 5 networks, with their aim, individual strengths and capabilities with geographical locations of the hospitals and catchment areas
EHRMPS Of Sites
|
S.No |
Name of Lead sites |
Clinical Trail Network |
Environmental and Health Risk Management Plan (EHRMP) |
|
1 |
Amrita Institute of Medical Sciences, Kochi, Kerala |
Ophthalmology |
Click Here |
|
2 |
Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry |
Oncology |
Click Here |
|
3 |
Gokula Education Foundation-M.S. Ramaiah Medical College and Hospitals, Bangalore |
Diabetology |
Click Here |
|
4 |
Tata Memorial Hospital, Mumbai |
Oncology |
Under process |
|
5 |
Medanta Institute of Education and Research, Haryana |
Rheumatology |
Under process |